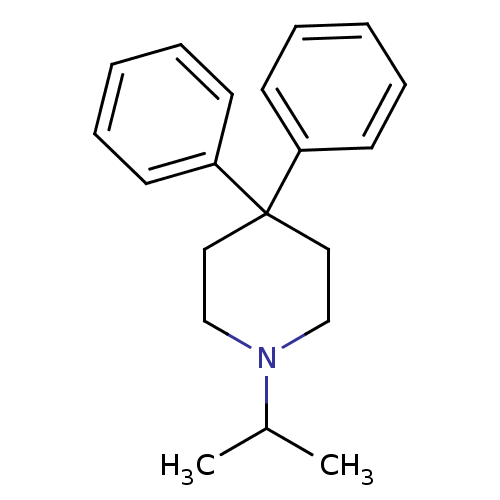
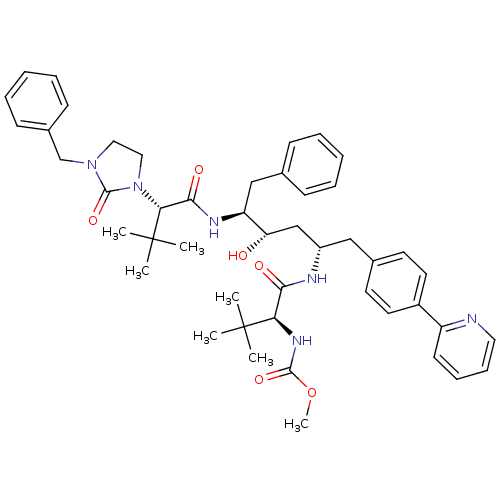
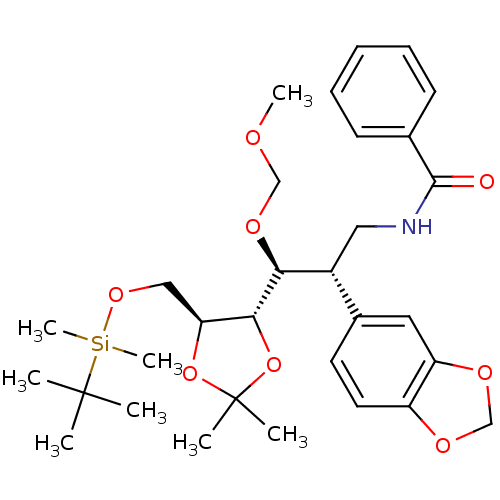
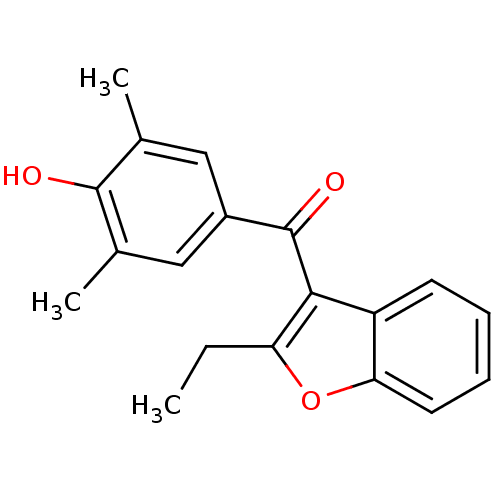
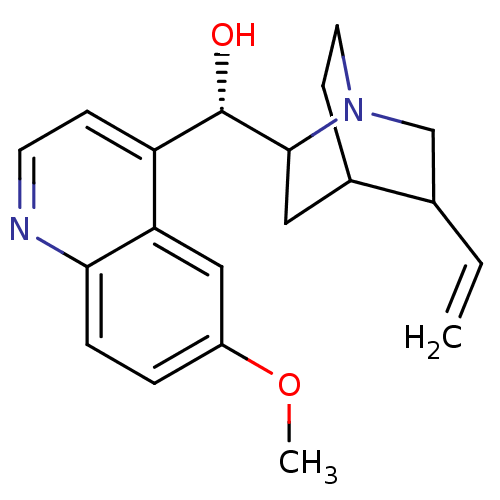
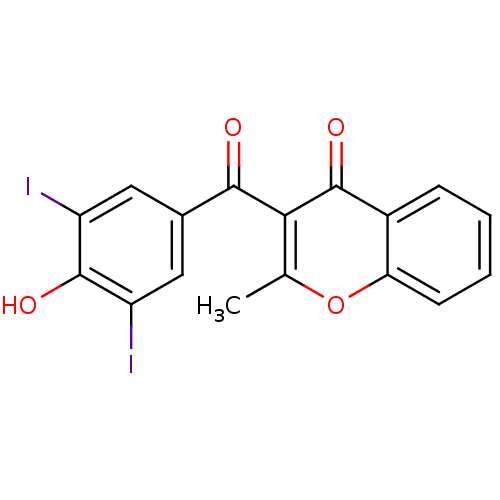
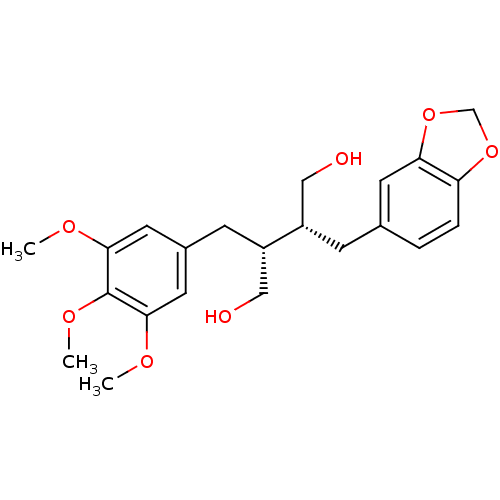
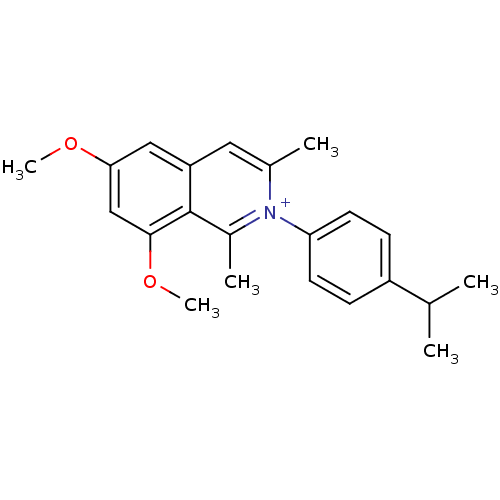
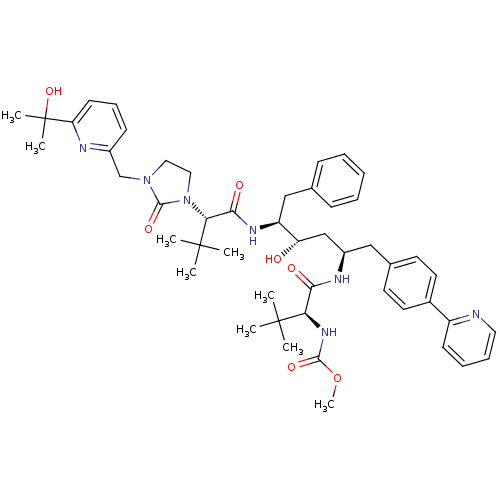
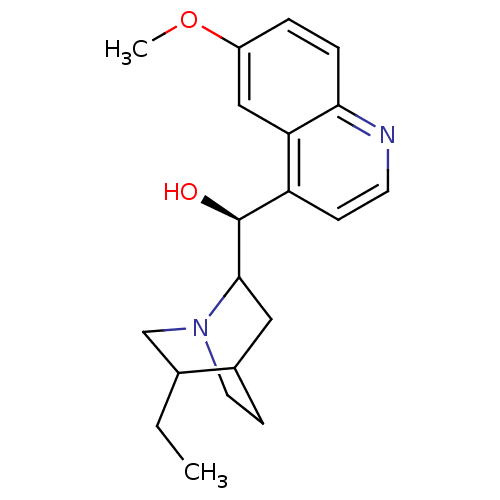
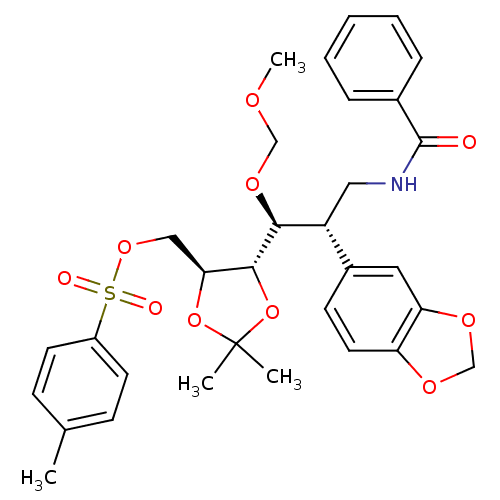
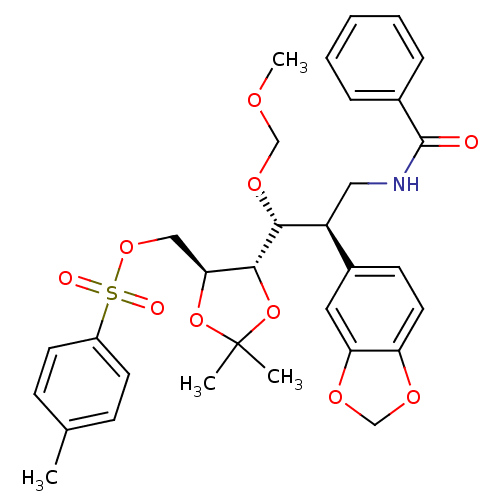
Affinity DataKi: 0.0220nMAssay Description:Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysisMore data for this Ligand-Target Pair
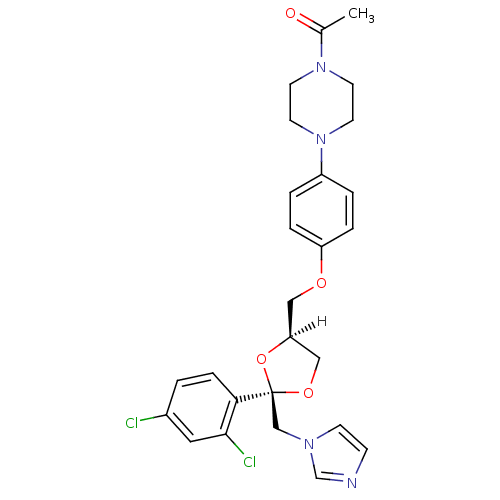
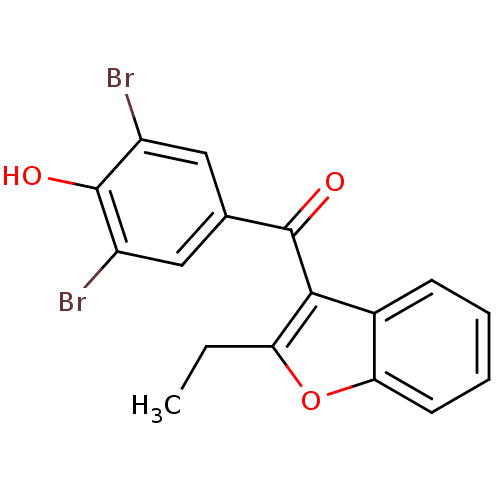
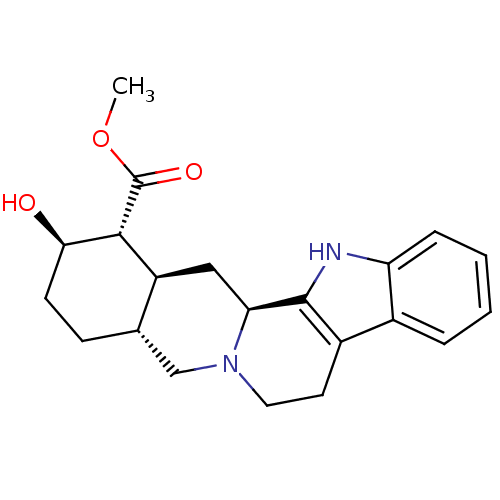
Affinity DataKi: 0.0260nMAssay Description:Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysisMore data for this Ligand-Target Pair
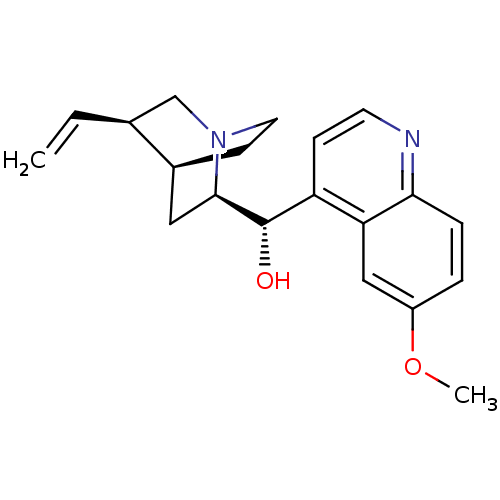
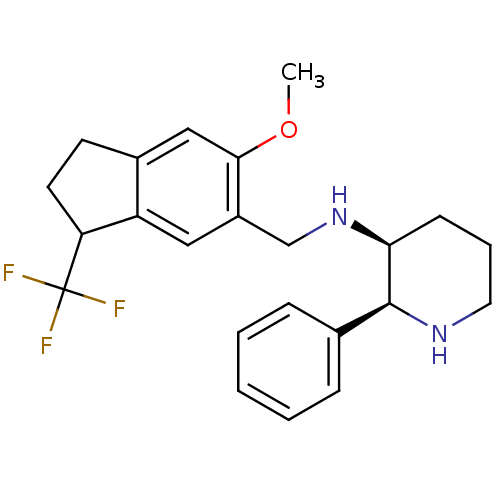
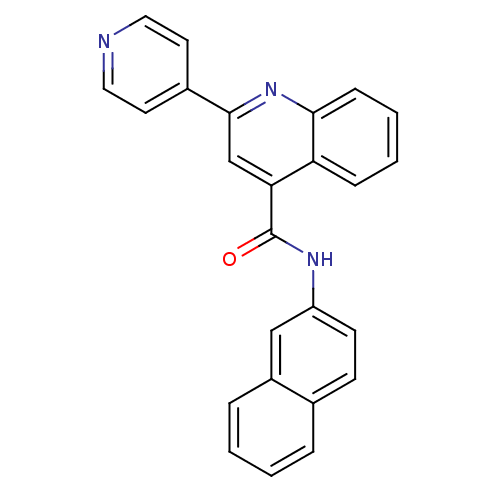
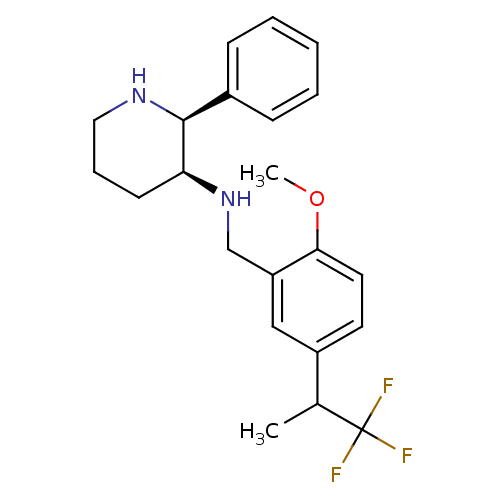
Affinity DataKi: 3.30nMAssay Description:Inhibitory constant for cytochrome P450 2D6More data for this Ligand-Target Pair
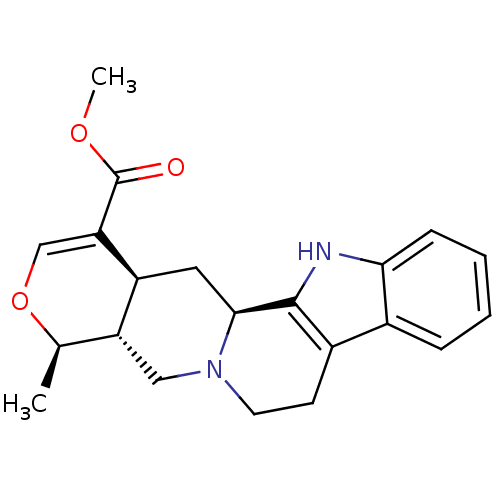
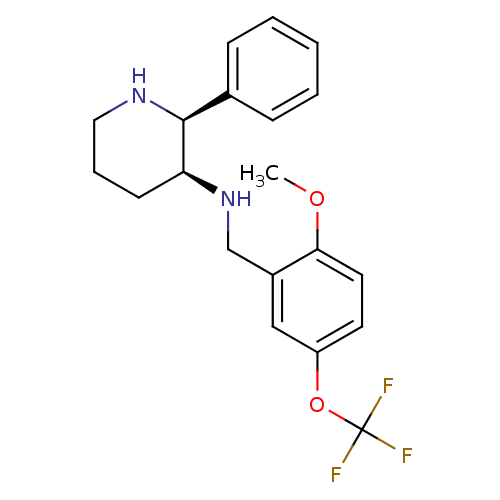
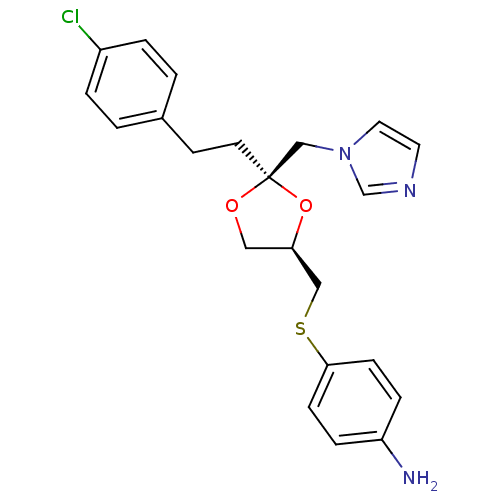
Affinity DataKi: 3.5nMAssay Description:Inhibition of 1'-hydroxybufuralol formation by human liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Binding affinity to CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Apparent inhibitory constant (Ki) for Bufuralol 1'-hydroxylation by human liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Inhibition of 1'-hydroxybufuralol formation by human liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 4.80nMAssay Description:Inhibitory constant for cytochrome P450 2D6More data for this Ligand-Target Pair
Affinity DataKi: 5.70nMAssay Description:Inhibition of partially purified cytochrome P450 2D6 1'-hydroxybufuralol formationMore data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysisMore data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Inhibition of reconstituted CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 9.80nMAssay Description:Inhibition of human CYP2D6 by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysisMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of reconstituted CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Inhibition of reconstituted CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of 1'-hydroxybufuralol formation by human liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibition of 1'-hydroxybufuralol formation by human liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant)More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Binding affinity to CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Binding affinity to human CYP2D6 using bufuralol as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Binding affinity to human CYP2D6 using bufuralol as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Binding affinity to CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Binding affinity for progesterone 6-beta-hydroxylase of hepatic microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 28.1nMAssay Description:Inhibition of human CYP2D6 by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline to 7-hydroxyquinoline measured every 15 mins by fluoresc...More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Binding affinity for cytochrome P450 2D6More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-hydroxyquinoline to 7-benzyloxyquinoline measured every minute for 15 mins ...More data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant)More data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Inhibition of reconstituted CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Binding affinity for progesterone 6-beta-hydroxylase of hepatic microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Inhibition of 1'-hydroxybufuralol formation by human liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Binding affinity to CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Inhibition of partially purified cytochrome P450 2D6 1'-hydroxybufuralol formationMore data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Inhibition of partially purified cytochrome P450 2D6 1'-hydroxybufuralol formationMore data for this Ligand-Target Pair
Affinity DataKi: 44.3nMAssay Description:Binding affinity to CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 49nMAssay Description:Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysisMore data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycinMore data for this Ligand-Target Pair
Affinity DataKi: 54.7nMAssay Description:Inhibition of human CYP2D6 by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant)More data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-hydroxyquinoline to 7-benzyloxyquinoline measured every minute for 15 mins ...More data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-benzyloxyquinoline to 7-hydroxyquinoline measured every 15 mins by fluoresc...More data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Binding affinity to human CYP2D6 using bufuralol as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 78nMAssay Description:Competitive inhibition of CYP2D6 (unknown origin) using dextromethorphan substrateMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant)More data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant)More data for this Ligand-Target Pair


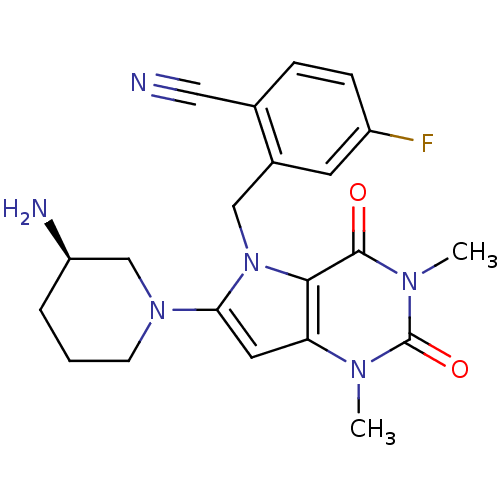
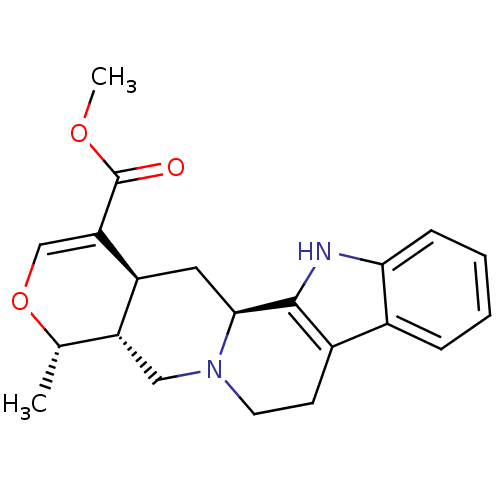
 3D Structure (crystal)
3D Structure (crystal)